Versorgungsforschungsprojekte unserer Mitgliedslabore
Laboratory reform counteracts the WHO hepatitis C elimination strategy in Germany
Jan Kramer1,2, Ingmar Wolffram3, Uli Früh4, Olaf Bätz2, Thomas Berg5, Johannes Wiegand5
1) Accredited Laboratories in Medicine (ALM) e.V., Invalidenstraße 113, 10115 Berlin; 2) LADR Laboratory Group Dr. Kramer & Colleagues, Lauenburger Str. 67, 21502 Geesthacht; 3) Südstadtpraxis Paderborn, Querweg 47, 33098 Paderborn; 4) WCG Consulting, Obere Wässere 1, 72764 Reutlingen; 5) Klinik für Gastroenterologie, Universität Leipzig, 04103 Leipzig
After the invention of direct antiviral agents (DAA) to treat chronic hepatitis C infection the World Health Organization has announced an initiative to eliminate the infection until the year 2030 (1). This initiative has been adapted to Europe and Germany by the HCV Elimination Manifesto and the national “BIS 2030 Strategie” of the German Government (2, 3). To eliminate HCV infection, two major principles must be fulfilled: (i) adequate early laboratory testing to detect so far undiagnosed infections and (ii) adequate treatment with direct antiviral agents (4).
Ganzen Beitrag lesen
Laboratory reform counteracts the WHO hepatitis C elimination strategy in Germany
Kramer et al., J Viral Hepat. 2019 Dec;26(12):1493-1495. doi: 10.1111/jvh.13188. Epub 2019 Aug 22.
Jan Kramer1,2, Ingmar Wolffram3, Uli Früh4, Olaf Bätz2, Thomas Berg5, Johannes Wiegand5
1) Accredited Laboratories in Medicine (ALM) e.V., Invalidenstraße 113, 10115 Berlin; 2) LADR Laboratory Group Dr. Kramer & Colleagues, Lauenburger Str. 67, 21502 Geesthacht; 3) Querweg 47, 33098 Paderborn; 4) WCG Consulting, Obere Wässere 1, 72764 Reutlingen; 5) Klinik für Gastroenterologie, Universität Leipzig, 04103 Leipzig
Address of correspondence:
Prof. Dr. Johannes Wiegand
Universität Leipzig, Klinik für Gastroenterologie
Liebigstr. 20
04103 Leipzig
Tel.: +49-341-9712330
Fax: +49-3419712339
After the invention of direct antiviral agents (DAA) to treat chronic hepatitis C infection the World Health Organization has announced an initiative to eliminate the infection until the year 2030 (1). This initiative has been adapted to Europe and Germany by the HCV Elimination Manifesto and the national “BIS 2030 Strategie” of the German Government (2, 3). To eliminate HCV infection, two major principles must be fulfilled:
(i) adequate early laboratory testing to detect so far undiagnosed infections and
(ii) adequate treatment with direct antiviral agents (4).
The latter is already possible in Germany, because the German health care system allowed reimbursement of all different DAA regimens after marketing authorization without major restrictions which induced a dramatic decline of patients listed for liver transplantation due to HCV induced cirrhosis (5) and has shifted the spectrum of HCV infected patients to early disease stages with absent or minimal fibrosis (6).
However, adequate screening and detection of so far undiagnosed infections still need improvement. Early detection strategies in the primary care setting, which is the most important entry level to the health care system in Germany, have recently been evaluated and rely on the testing of anti-HCV in serum as screening parameter for risk patients and patients with elevated aminotransferases (7). In the past, anti-HCV testing was extra-budgetary reimbursed by statuary health care insurances and not taken into account when the laboratory budget of each outpatient physician was estimated. This policy has changed since 1st April 2018: Here, a nationwide laboratory reform initiated by the Association of Statutory Health Insurance Physicians(“Kassenärztliche Bundesvereinigung (KBV)”) was established which should reduce the demands of blood samples and because of that the laboratory costs of patients insured in the statuary health care insurances.
The reform determines lower and high laboratory costs per lab-order for each medical group (e. g. for primary care physicians, internal medicine doctors, gynecologists, etc.) in the outpatient field. These values are specified by the KBV and they are fixed for a longer time. If an individual outpatient physician has laboratory costs below the lower value of his peer-group (i. e. a primary care physician in the group of primary care physicians), a gratuity will be paid 100 %. If an individual outpatient physician has laboratory costs over the high value of his peer-group, a gratuity will not be paid (0 %).If the lab costs are between the lower and high value, the gratification is paid pro rata.
Thus, it is attractive to order a priori as few laboratory tests as possible in order to be below the mean laboratory costs of the peer group at the end of the reference period. In addition to this policy, the reimbursement strategy of the anti-HCV test was changed: Now, anti-HCV in serum (code: EBM 32618) is not an extra budgetary test any more, but is included in the laboratory budget and is therefore relevant for the budget calculation. In contrast to anti-HCV, the status of HBsAg as screening parameter for chronic hepatitis B infection has not been changed, and HBsAg is still extra-budgetary (code: EBM 32781). We therefore analyzed the impact of the laboratory reform on the order of anti-HCV tests in large laboratory institutes.
“ALM e.V.” is the professional association of Accredited Laboratories in Medicine in Germany (www.alm-ev.de) and includes > 200 medical laboratories. We analyzed the number of anti-HCV tests (code: EBM 32618) in 510.656 anonymized patient data sets of the quarters I/2018 (the quarter before the initiation of the laboratory reform), and II and III/2018 (the first two quarters after the initiation of the laboratory reform), in comparison to the number of anti-HCV tests in the quarters I–III of the year 2017. Due to the number of participating laboratories and the number of analyzed patient data sets the results are considered to be representative for Germany.
The number of anti-HCV tests ordered by physicians of the outpatient setting declined by 9.4 % in quarters I–III 2018 in comparison to the same time period of the year 2017 (figure 1). The number of tests declined already in quarter I/2018, because announcements on potential economical disadvantages were released prior to the start of the laboratory reform. The number of ordered HBsAg tests has declined by 7.4 % in the same observation period, indicating that the reduced anti-HCV laboratory orders are not parameter-specific, but most likely a surrogate of the intention of the laboratory reform to generally lower the demands of blood samples and laboratory costs.
Thus, the laboratory reform impairs successful hepatitis screening strategies and might also affect the regulatory reporting of HCV infections to the National Institute of Infectious Diseases (Robert Koch Institut). The German Society of Gastroenterology (DGVS) has addressed this issue to the Association of Statutory Health Insurance Physicians and demands that at least the anti-HCV test is excluded again from the laboratory reform and re-classified as an extra-budgetary screening parameter. If the current policy is not changed, the laboratory reform directly counteracts the WHO hepatitis C elimination strategy in Germany.
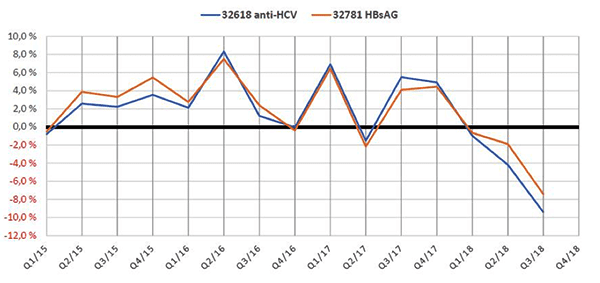
Figure 1: Relative number of anti-HCV (EBM code 32618) and HBsAg (EBM code 32781) laboratory requisitions in comparison to the quarter of the preceding year
References
- World Health Organization. Global Hepatitis Report 2017. Geneva: World Health Organization 2017.
- Papatheodoridis GV, Hatzakis A, Cholongitas E, Baptista-Leite R, Baskozos I, Chhatwal J, et al.
Hepatitis C: The beginning of the end-key elements for successful European and national strategies to eliminate HCV in Europe. J Viral Hepat 2018 Mar;25 Suppl 1:6-17. - Bundesministerium für Gesundheit, Bundesministerium für wirtschaftliche Zusammenarbeit und Entwicklung.
BIS 2030 – Strategie zur Eindämmung von HIV, Hepatitis B und C und anderen sexuell übertragbaren Infektionen. Bundesministerium für Gesundheit Referat Öffentlichkeitsarbeit Berlin 2016 Apr 6. - Wedemeyer H, Duberg AS, Buti M, Rosenberg WM, Frankova S, Esmat G, et al.
Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat 2014 May;21 Suppl 1:60-89. - Herzer K, Gerken G, Kroy D, Tacke F, Plewe J, Eurich D, et al.
Impact of direct-acting antiviral therapy on the need for liver transplantation related to hepatitis C in Germany.
J Hepatol 2018 Oct;69(4):982-984. - Huppe D, Serfert Y, Buggisch P, Mauss S, Boker KHW, Muller T, et al.
[4 years of direct-acting antivirals (DAAs) in the German Hepatitis C-Registry (DHC-R)].
Z Gastroenterol 2019 Jan;57(1):27-36. - Wolffram I, Petroff D, Batz O, Jedrysiak K, Kramer J, Tenckhoff H, et al.
Prevalence of elevated ALT values, HBsAg, and anti-HCV in the primary care setting and evaluation of guideline defined hepatitis risk scenarios. J Hepatol 2015 Jun;62(6):1256-1264.
Prävention von Darmkrebserkrankungen
Bericht der Akkreditierten Labore in der Medizin über den Einsatz immunologischer fäkaler okkulter Bluttests
Prävention und Gesundheitsförderung (PUGE), Springer Verlag, Februar 2019
Prof. Dr. Jan Kramer, Uli Früh
Hintergrund: Kolorektale Karzinome (KRK) sind weltweit die dritthäufigste Krebserkrankung. In Deutschland erkrankten im Jahr 2013 neu 28.360 Frauen und 34.050 Männer bei insgesamt 25.693 durch ein KRK begründeten Todesfällen. Die Inzidenz steigt ab dem 50. Lebensjahr an. Durch ein präventives Screening ab diesem Alter sollen Adenome als Vorstufen oder KRK frühzeitig entdeckt werden. Die Inzidenz von Dickdarmkrebs sinkt in Deutschland seit 2004, während fortgeschrittene Vorstufen häufiger durch die im Jahr 2002 eingeführten Früherkennungskoloskopien detektiert werden. Wird die Koloskopie abgelehnt, kann der sog. fäkale okkulte Bluttest (FOBT) im Stuhl eingesetzt werden …
Diagnostik der Anämie und des Eisenmangels in der Schwangerschaft
Eine Analyse auf der Basis von Labordaten
Der Frauenarzt, Dezember 2016, Seite 1146 – 1155
Wolf Kirschner, Hans-Ulrich Altenkirch, Joachim W. Dudenhausen, R. Neuber, Michael Müller, Lothar Röcker, A. Kunz
Eisenmangel und Eisenmangelanämie in der Schwangerschaft sind häufig und relevant, da sie mit negativen Folgen für Mutter und Kind assoziiert sind. In den Mutterschaftsrichtlinien ist eine Bestimmung des Hb-Wertes vorgesehen, ansonsten existieren keine einheitlichen Empfehlungen zum Screening und zum strukturierten Vorgehen bei Eisenmangel. Nur die schwerste Form des Eisenmangels, die Anämie, lässt sich am Hb erkennen, während bei Speichereisenmangel und eisendefizienter Erythropoese der Hb noch unauffällig, aber das Ferritin bereits vermindert ist. Welche eisendiagnostischen Laborparameter niedergelassene Frauenärzte wie häufig anfordern, war bisher unbekannt. Die vorliegende Arbeit analysierte daher Labordaten eines Berliner Labors und Surveydaten eines Interventionsprogramms zur Senkung der Frühgeburtlichkeit.
Epidemiologische Untersuchung zur Häufigkeit eines Vitamin-D-Mangels in Norddeutschland
Kramer J, Diehl A, Lehnert H.
Dtsch Med Wochenschr. 2014 Mar;139(10):470-5.
Hintergrund und Fragestellung
Die für eine ausreichende Vitamin-D-Produktion empfohlene Sonnenlichtexposition von 30 Minuten pro Tag wird kaum erreicht. Begründet ist dies vor allem in den Witterungsbedingungen sowie heutigen Lebens- und Arbeitsgewohnheiten. Das Ziel unserer Studie war, das Ausmaß eines Vitamin- D-Mangels in Norddeutschland zu untersuchen.
Methodik
Hierzu wurden retrospektiv die 25-Hydroxy-Vitamin-D-Spiegel von über 99 000 Menschen aus Norddeutschland der Jahre 2008–2011 nach Alter, Geschlecht und Jahreszeit ausgewertet. Eine Einteilung des 25-Vitamin-D-Status erfolgte in suffiziente (> 75 nmol/l) und insuffiziente (50 – 75 nmol/l) Versorgung sowie Vitamin-D-Mangel (< 50 – 27,5 nmol/l) und schweren Vitamin-D-Mangel (< 27,5 nmol/l).
Ergebnisse
Eine Vitamin-D-Unterversorgung konnte in allen Altersgruppen sowohl bei Frauen als auch Männern in Norddeutschland nachgewiesen werden. In den sonnenarmen Monaten war der Vitamin-D-Mangel besonders ausgeprägt. So zeigten in den Monaten Januar bis April mehr als 30 % der untersuchten Personen einen schweren Vitamin-D-Mangel. Die Untersuchung ergab zudem, dass nahezu gleichverteilt über die einzelnen Monate des gesamten Jahres 25-Vitamin- D-Bestimmungen durchgeführt wurden. Insgesamt wurde häufiger bei Älteren als bei Jüngeren diese Untersuchung angefordert. Allerdings konnte gerade auch für das Jugend- und junge Erwachsenenalter ein schwerer Vitamin-D-Mangel bei ca. 25 % der untersuchten Personen detektiert werden.
Folgerungen
Aus den dargestellten Ergebnissen kann die Empfehlung abgeleitet werden, einmal pro Jahr 25-Vitamin-D-Spiegel in den Monaten Januar bis April zu bestimmen, um rechtzeitig einen schweren Mangel zu erkennen und präventiv-therapeutisch eingreifen zu können.
Prevalence of elevated ALT values, HBsAg, and anti-HCV in the primary care setting and evaluation of guideline defined hepatitis risk scenarios.
J Hepatol. 2015 Jun;62(6):1256-64. doi: 10.1016/j.jhep.2015.01.011. Epub 2015 Jan 21
Wolffram I1, Petroff D2, Bätz O3, Jedrysiak K3, Kramer J3, Tenckhoff H4, Berg T4, Wiegand J5; German Check-Up 35+ Study Group
Background and aims
Prevalence data for hepatitis B and C and an evaluation of a guideline based screening in the primary care setting are not yet available. We therefore implemented a hepatitis B surface antigen (HBsAg) and anti-hepatitis C virus (HCV) screening and developed guideline based screening strategies.
Methods
HBsAg, anti-HCV, and alanine aminotransferase (ALT) were included in a routine check-up together with a questionnaire covering 16 guideline adapted risk scenarios. Significant risk factors were identified by stepwise logistic regression.
Results
51 private practices screened 21,008 patients. The HBsAg, anti-HCV, and HCV-RNA prevalence was 0.52%, 0.95%, and 0.43%, respectively. Infections were previously unknown in 85% and 65% of HBsAg and anti-HCV positive individuals, respectively. Sexual risk factors were under-reported, while the following scenarios were significantly associated with viral infections (Odds ratio [95% confidence interval]). HBV: Immigration (4.4 [2.9, 6.7]), infection in household (2.5 [1.2, 4.5]), male gender (1.6 [1.1, 2.4]). Male immigrants had a 2.1% HBsAg prevalence and 80% were unaware of the infection. HCV: IV drug use (384 [233, 644]), blood transfusion before 1992 (5.3 [3.5, 7.9]), immigration (2.4 [1.5, 3.6]). Presence of either one of the HBV related guideline defined risk scenarios or elevated ALT identified 82% of previously undiagnosed patients. Presence of one of the three significant HCV risk factors or elevated ALT levels diagnosed 83% of unknown HCV-RNA positive cases by screening only 26% of the population.
Conclusions
Undiagnosed hepatitis B and C infections frequently exist in the primary care setting. Easy to apply guideline defined risk scenarios help to diagnose previously unknown infections.